Department of Chemistry,
University of Oxford,
Inorganic Chemistry Laboratory,
South Parks Road, OX1 3QR
kylie.vincent@chem.ox.ac.uk

A wide range of micro-organisms express hydrogenases - highly active enzymes for oxidation of H2, or reduction of H+ to produce H2. The efficiency of H2/H+ interconversion by hydrogenases is providing inspiration for development of new catalysts for fuel cells or clean H2 production built from abundant metals, but this requires an intimate knowledge of how H2 is activated at hydrogenase active sites. The Vincent group are using a combination of electrochemical and spectroscopic methods to understand hydrogenase active site chemistry.
Direct electrochemical methods, in which hydrogenases are adsorbed as a sub-monolayer film onto a graphite electrode, provide precise control over active site chemistry. In this approach called (protein film electrochemistry), adsorbed enzyme molecules exchange electrons directly with the electrode so that no electron-transfer mediators are required. The current at the electrode reports on the catalytic activity of the adsorbed hydrogenase at each applied potential. Protein film electrochemistry has provided much insight into the chemistry of hydrogenases.
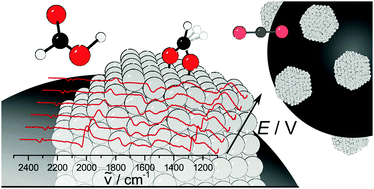
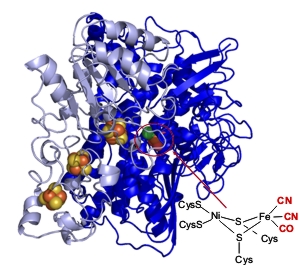
We are using a range of coupled electrochemical and infrared spectroscopic approaches to reveal structural details of enzyme active site chemistry during catalytic turnover. The active sites of hydrogenases incorporate ligands that are unusual in biology: carbon monoxide (CO) and cyanide (CN-). These ligands provide a useful spectroscopic handle for studying structural changes at the metal centre because vibrational transitions associated with CO and CN- give rise to relatively intense absorption bands in the infrared.
We have developed a series of applications of hydrogenases for H2-driven biocatalysis. One of these, the HydRegen catalyst system, uses enzymes for both H2-driven NADH recycling and a selective NADH-dependent biotransformation 'plugged' into carbon particles. We have also developed systems for H2-driven recycling of reduced flavins with applications in catalysis by ene reductases and nitro reductases. We are working on a range of chemo-bio catalyst systems. For these projects we have close industrial collaborations as well as many academic collaborators.
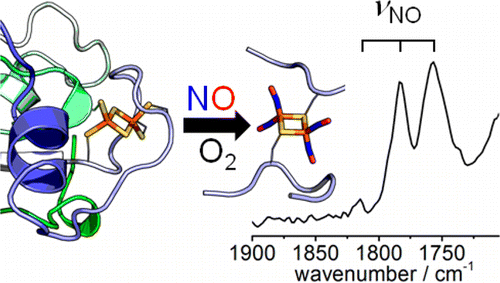
We are interested in fundamental principles behind the binding of small molecule ligands to metal centres. Interests include reactions of nitric oxide with protein-bound iron sulfur clusters (with relevance to bacterial sensing of NO) and interactions of CO with iron sulfur clusters.
We have applied IR spectroelectrochemistry to examine surface chemistry and poisoning of supported nanoparticles of platinum and other precious metals in fuel cell electrocatalysis, under fuel flow conditions. We are working with academic collaborators on a range of other mechanistic projects in energy catalysis.