New approaches to coupling infrared spectroscopy and electrochemistry
We have developed
IR spectroelectrochemical methods to address catalysts supported on carbon particles assembled into high surface area 3D electrodes. This research is supported by a
major ERC Starting Grant.ref
-Understanding active site chemistry of hydrogenase enzymes
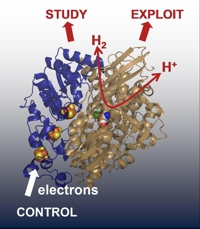
A wide range of micro-organisms express hydrogenases - highly active
enzymes for oxidation of H2, or reduction of
H+ to produce H2. The efficiency of
H2/H+ interconversion by hydrogenases is providing inspiration for development of new catalysts for fuel cells or
clean H2 production built from abundant metals, but this requires an intimate knowledge of how H2 is activated at hydrogenase active sites. The Vincent group are using a combination of electrochemical and spectroscopic methods to understand hydrogenase active site chemistry.
Direct electrochemical methods, in which
hydrogenases are adsorbed as a sub-monolayer film onto a graphite electrode,
provide precise control over active site chemistry. In this approach called (protein
film electrochemistry), adsorbed enzyme molecules exchange electrons
directly with the electrode so that no electron-transfer mediators are
required. The current at the electrode reports on the catalytic activity of the adsorbed
hydrogenase at each applied potential. Protein film electrochemistry has provided much insight into the
chemistry of hydrogenases.
The Vincent group has established a method for combining protein film electrochemistry at carbon electrodes with infrared spectroscopy. We call this approach 'protein film infrared electrochemistry' (PFIRE), and we are using this to reveal structural details of enzyme active site chemistry during catalytic turnover on an electrode. The active sites of
hydrogenases incorporate ligands that are unusual in biology:
carbon monoxide (CO) and cyanide (CN-). These
ligands provide a useful spectroscopic handle for studying structural
changes at the metal centre because vibrational transitions associated
with CO and CN- give rise to relatively intense
absorption bands in the infrared. Using the PFIRE approach we have obtained strong evidence for the role of a Ni(I) active site called Ni-L in catalytic turnover of E. coli NiFe hydrogenase I.ref
|